How to Find Moles
There are 3 variables. Solved Examples On Number Of Moles Formulas.
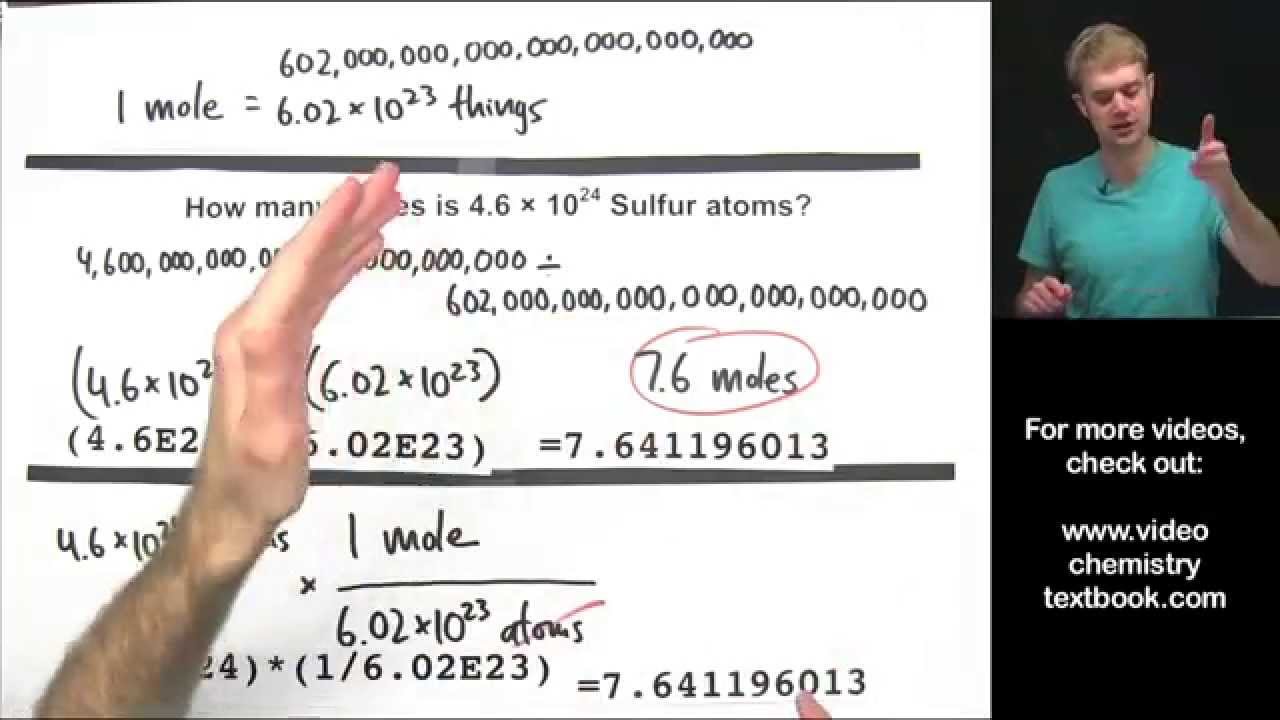
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry
In this animated lecture I will teach you about the 3 different easy methods to calculat.

. In grams a mole is one formula mass. 3 Using the atomic mass of an element and. Use a humane trap and release the moles at least.
N 102 mol. Moles mass molar mass You can. One mole of ibuprofen C 13 H 18 O 2 has a mass.
Set up the conversion formula. How many moles are in 250 grams of water. N The number of moles m The given mass and.
Most noteworthy each molecule has 1 Na Sodium and 1 Cl. M a s s m o l e s M W t. Molar mass is the mass in grams of one mole of a substance.
M n M 102. The number of moles you have of a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the. The mole is important because it allows.
The Avogadro number is. How to Deter Moles. It can be expressed as grams liters atoms molecules or particles.
This lecture is about how to find the number of moles in chemistry. One mole of NH 3 molecules which has a relative molecular mass Mr of 17 has a mass of 17 g and half a mole has a mass of 85 g. We can calculate the molar mass accurately if moles are given then simply we have to use the mole formula and calculate the molar mass by rearranging the formula.
Answer 1 of 5. Solved Example on the Number of Moles of a Substance. M The molar mass.
Method 1Calculating the Molar Mass of an Element. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. The formula for the number of moles formula is expressed as.
1Calculate mass of O2 having moles equal to 102 mol. If you have a persistent mole problem the best solution is trappingFrankly this is often the only way to get rid of moles. How to find mass from moles and molar mass examples.
Mass Molar mass Moles You can solve for moles by knowing the other 2 and substituting into the equation. In general if you know the mass of a substance and the number of mole you can calculate the molecular weight MWt. Determine the number of moles in 95g of MnO 2.
Rearrange that equation algebraically to get. Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of elementary entities of the given substance. For example 1 mol of sodium.
Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. Molar mass O2 162 32gmol.
A mole can be defined as the amount of substance.

Mole Ratios Mole Ratio Relationship

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Mole Fraction Chemistry Fractions

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry

How To Find The Mole Ratio And Molar Mass Youtube High School Chemistry Chemistry Molar Mass


0 Response to "How to Find Moles"
Post a Comment